All articles by Aninda Chakraborty
Flagship Pioneering forms biotech research alliance with CUHP and MTI
The collaboration will enable more than 40 companies in Flagship Pioneering’s ecosystem collaborate with CUHP and MTI
NHS England green lights quizartinib for blood cancer treatment
The treatment is expected to benefit patients newly diagnosed with Acute myeloid leukaemia (AML) increasing the chance of remission and boosting long-term survival
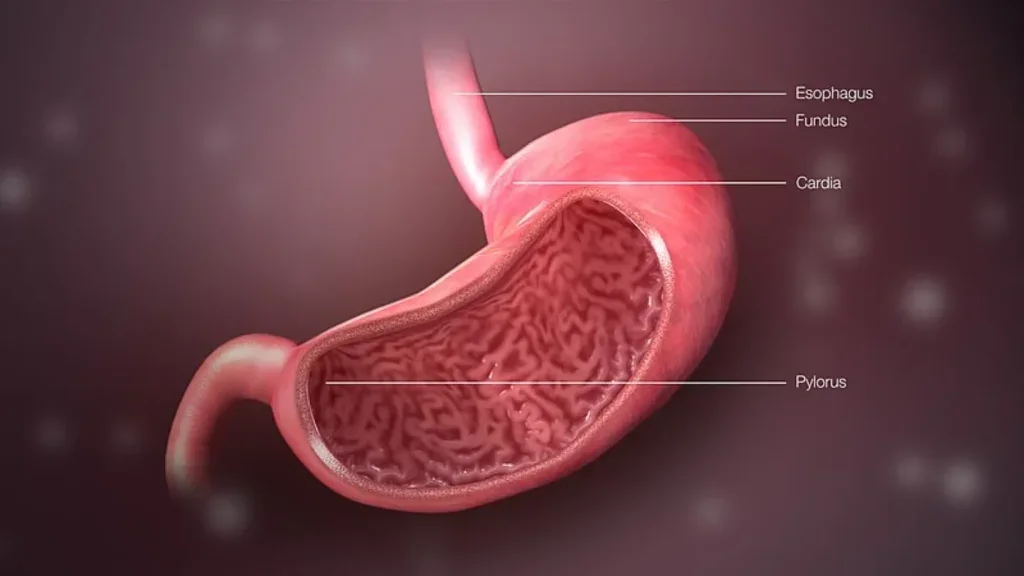
Vanda’s tradipitant fails to secure FDA approval for gastroparesis
Gastroparesis condition shows down gastric emptying and causes severe nausea, vomiting, and difficulty finishing a normal meal among other symptoms
Novartis, Versant establish new biotechnology firm Borealis Biosciences
Borealis started its journey with more than $150m in combined Series A financing from Novartis and Versant
VION buys lipid-based excipients supplier Echelon Biosciences
Salt Lake City-based Echelon provides lipid synthesis and the engineering of lipid nanoparticles
RedHill Biopharma launches Talicia in UAE to treat H. pylori infection
In the Phase 3 study, Talicia reported 84% eradication of H. pylori infection in the intent-to-treat (ITT) group compared to 58% in the active comparator arm
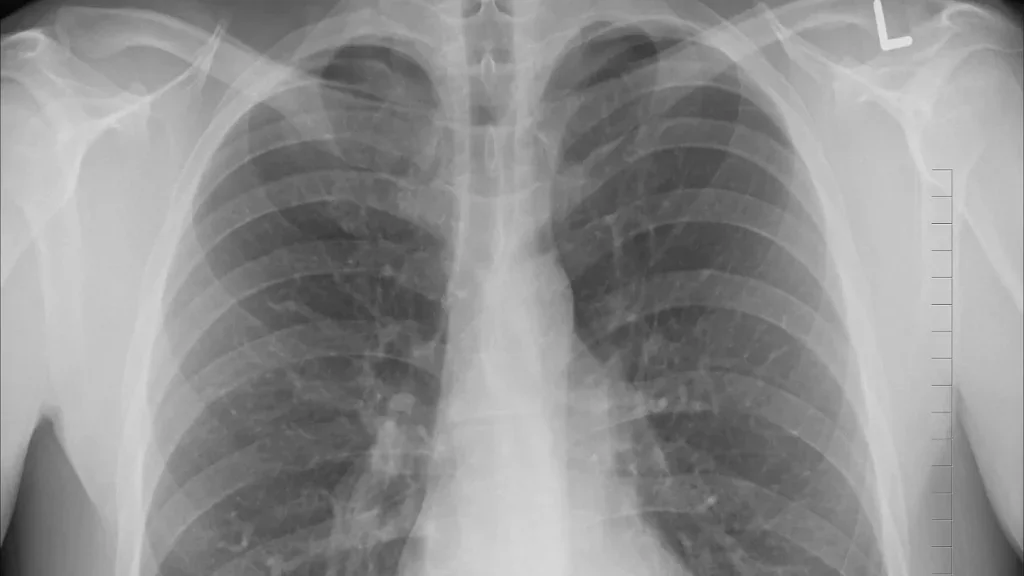
China’s NMPA approves Dupert (Fulzerasib) for advanced NSCLC
Innovent entered into an exclusive licence agreement with GenFleet Therapeutics in September 2021 for the development and commercialisation of fulzerasib in China
Kiromic BioPharma reports interim results of NSCLC treatment Phase I trial
The clinical site has also reported no dose limiting toxicities (DLTs) for patients who underwent the full course of therapy
US FDA declines to approve Lykos’ midomafetamine for PTSD
MDMA, an entactogen, is seen to support psychotherapy by reducing the brain’s fear response enabling the patients to become more resilient to painful memories
Ultimovacs’ cancer vaccine fails in meet endpoints in Phase II FOCUS trial
The FOCUS investigator-initiated randomised Phase II clinical trial was initiated to assess the efficacy of UV1 vaccination, in combination with pembrolizumab