All articles by
US FDA approves Italfarmaco’s Duvyzat to treat DMD in children and adults
The FDA approval is supported by the results from EPIDYS, a randomized, double-blind, placebo-controlled 18-month Phase 3 clinical trial, which met its primary endpoint, and favourable results on key secondary endpoints
Lonza to buy Genentech’s biologics manufacturing site in California for $1.2bn
The deal is anticipated to boost Lonza’s large-scale biologics manufacturing capacity and will also help the firm cope with the demand for commercial mammalian contract manufacturing
AstraZeneca to acquire Canadian cancer drugmaker Fusion for $2bn
The transaction complements AstraZeneca’s oncology portfolio with Fusion’s pipeline of RCs, including its lead programme FPI-2265, and replaces the traditional chemotherapy and radiotherapy with more targeted treatments
Microbix’s Clot-Buster Drug Project Advances
Sequel Pharma Executes Agreement with CDMO for Drug Substance Production
Optinose gets FDA nod for Xhance in chronic rhinosinusitis without nasal polyps
Xhance is a drug-device combination product that utilises the Exhalation Delivery System engineered to administer a topical steroid to the intricate and deep regions of the nasal cavity crucial for sinus ventilation and drainage
AstraZeneca to acquire French drugmaker Amolyt Pharma for $1.05bn
AstraZeneca said that the acquisition will further expand the pipeline of Alexion, AstraZeneca Rare Disease, beyond complement inhibition, building on its success in bone metabolism and opportunity in rare endocrinology
Pearl Bio teams up with Merck to discover novel engineered biologics
The collaboration will initially focus on the discovery and development of biologic therapies for the treatment of cancer using Pearl’s exclusive GRO technology, which facilitates working in both cell-based and cell-free systems
Dassault Systèmes to support CDR-Life in development of cancer therapies
Under the partnership, Dassault Systèmes, a science-based firm with virtual twin experiences, and CDR-Life will study the stability of antibody-based biologics known as T-cell engagers
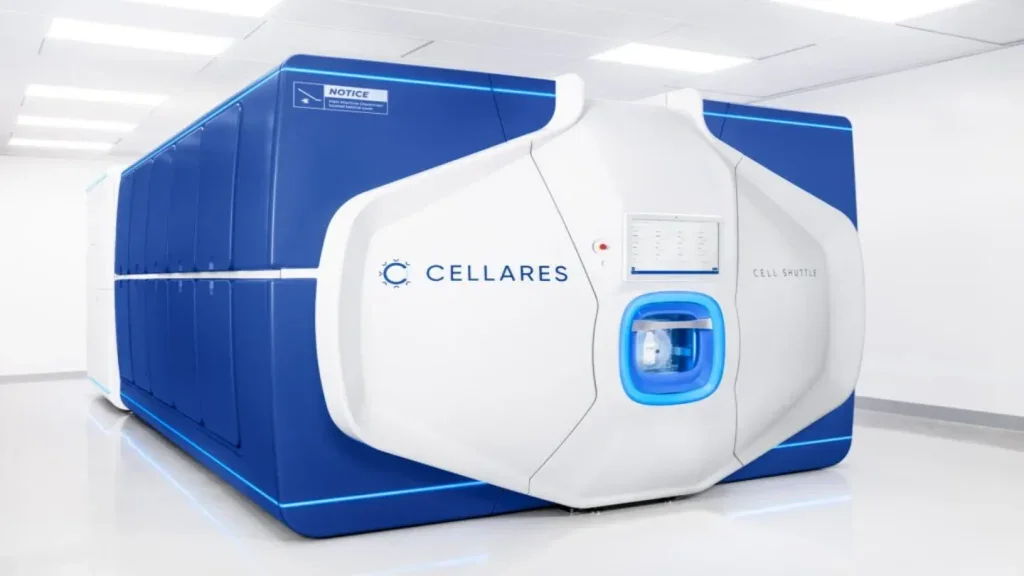
Cellares introduces cGMP-compliant Cell Shuttle for cell therapy manufacturing
The Cell Shuttle is an automated, ultra-high throughput, manufacturing platform designed to address the global patient demand while lowering process failure rates and costs
GV20 Therapeutics Joins NVIDIA Inception to Develop AI Models for Drug and Target Discovery
GV20 Therapeutics will leverage the resources provided by NVIDIA Inception to develop new AI models for drug discovery, focused on novel therapeutic antibodies