Drug and consumer product technologies developer AptarGroup said that its N-Sorb nitrosamine mitigation solution has been accepted into the US Food and Drug Administration’s (FDA) Emerging Technology Program (ETP).
The programme aims to support new methods of pharmaceutical product design and manufacturing.
N-Sorb nitrosamine mitigation solution uses the 3-Phase Activ-Polymer platform technology of Aptar CSP Technologies, a unit of AptarGroup. The technology is designed to tackle N-nitrosamine impurities in pharmaceuticals.
These impurities are probable human carcinogens, raising regulatory concerns and leading to drug recalls.
Nitrosamines can form during storage or transport, which poses risks to patient health.
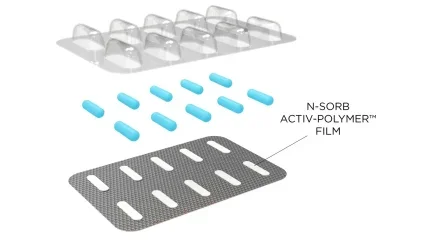
N-Sorb technology, launched in early 2020, can be deployed into various formats by combining active material science with polymers.
It can be incorporated into a blister pack that uses N-Sorb Activ-Film material in each Activ-Blister cavity.
This technology protects sensitive Active Pharmaceutical Ingredients (APIs) from moisture and oxygen.
It can also be used in container closure systems. N-Sorb reacts with nitrosamine precursors in packaging to inhibit nitrosamine formation and scavenge existing impurities after they have formed.
According to AptarGroup, the nitrosamine mitigation solution delivers Generally Recognised as Safe (GRAS) material directly in the packaging to avoid the need to reformulate drug products. This supports compliance with the US Food and Drug Administration (FDA) and European Union (EU) European Medicines Agency (EMA) regulations on safe nitrosamine levels.
The active packaging approach will help in managing impurities and degradation, enhancing overall mitigation strategies.
It also aligns with the recent FDA guidance, updated earlier this month.
Furthermore, N-Sorb technology can accelerate drug development and reduce shortages caused by recalls.
Aptar CSP Technologies general manager and global commercial operations vice president Badre Hammond said: “The FDA’s Emerging Technology Program is highly selective, reserved for the most promising pharma and healthcare sector solutions.
“Our ability to mitigate nitrosamine formation with active material science introduces a critical quality control element, designed to ensure patient safety.”
As part of the programme, industry representatives collaborate with the FDA’s Emerging Technology Team.
The members discuss and resolve potential technical and regulatory issues related to new technologies before regulatory submission.
The ETP intends to modernise the pharmaceutical industry and help reduce time and costs for introducing new solutions.






