Johnson & Johnson has agreed to acquire Numab Therapeutics’ fully owned subsidiary Yellow Jersey Therapeutics, which holds the rights to NM26, in a transaction worth up to $1.25bn.
Under the terms of the agreement, the US-based pharmaceutical company will acquire Yellow Jersey Therapeutics, which holds the rights to NM26.
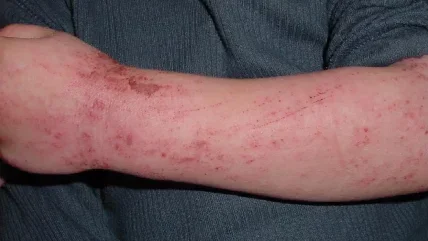
NM26 is a Phase 2-ready, investigational bispecific antibody that targets two clinically proven pathways in atopic dermatitis (AD), IL-4R alpha subunit (IL-4Rα) and IL-31.
The antibody was discovered and engineered using Numab’s unique MATCH technology platform, designed to fuel a new wave of multi-specific antibody drug candidates.
The US drugmaker will obtain full rights to develop, manufacture and commercialise NM26 worldwide, for the treatment of atopic dermatitis and follow-on indications.
The proposed acquisition is expected to be completed in the second half of 2024, subject to clearance under the Hart-Scott-Rodino Antitrust Improvements Act and other closing conditions.
Johnson & Johnson Innovative Medicine Global Immunology Therapeutic Area Head David Lee said: “To deliver durable, symptom-free remission for the millions of people living with AD, our medicines need to be tailored to target multiple disease-driving pathways in different patient subpopulations.
“That’s why we are committed to developing differentiated bispecifics that combine the targeting of two distinct disease-driving pathways.
“NM26 has the potential to deliver a treatment specifically for patients who have inflamed skin associated with intense itching.”
AD is the most common inflammatory skin disease and is highly heterogeneous with different disease-driving mechanisms in distinct patient subpopulations.
The patients experience inflamed skin with itch, and scratching causes skin pain and abrasions, along with infection, difficulty in sleeping, anxiety, stress, depression, and risk of suicide.
NM26 targets IL-4Rα, which triggers Th2-mediated skin inflammation, and IL-31, which impacts skin itch and subsequent scratching that worsen the disease.
In addition to AD, NM26 can also be effective for other inflammatory skin diseases involving Th2 inflammation and itch.
Morgan Stanley & Co. International and Centerview Partners served as financial advisors and Baker McKenzie as the legal advisor to Numab on this transaction.
Numab founder and CEO David Urech said: “We are thrilled to enter into this agreement with J&J and are confident they will be able to rapidly advance the development of NM26 for patients in need of a better treatment for atopic dermatitis and other conditions.
“This transaction validates the power of our discovery and engineering platform and its potential to bring multiple novel multi-specific antibodies to large, underserved patient populations.
“Our partnering strategy engaging biopharma partners such as Kaken, Eisai, Boehringer Ingelheim and Ono from early on, has been instrumental in realising the value of our platform and will continue to be key to advancing our potentially transformative immunology and oncology programs.”
Earlier this year, J&J agreed to acquire Proteologix, a US-based biotechnology company developing bispecific antibodies for immune-mediated diseases, for $850m.
Through the acquisition, J&J aims to advance its dermatology portfolio and expand its portfolio of bispecific antibody programmes for other diseases.






